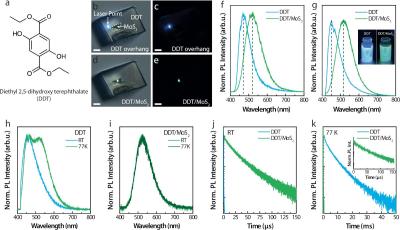
Researchers from the the University of Michigan developed a new class of phosphorescence OLED emitters that do not contain heavy metals. The metal was replaced with a new hybrid material. The researchers collaborated with colleagues from Inha University, and Sungkyunkwan University.
In current phosphorescence OLEDs, the emitters include a heavy metal, in most cases either iridium or platinum. These heavy metals generate a magnetic field that forces the same spin direction excited electron to turn quickly, resulting in faster light emission as it returns to its ground state. The researchers replaced the heavy metal with a 2D layer of molybdenum and sulfur near a similarly thin layer of the organic light emitting material, achieving the same effect by physical proximity without any chemical bonding.
This is an interesting research that could lead to a new class of commercial heavy-metal free phosphorescence OLED emitters, but it also holds a surprising result. When analyzing the new molecular hybrid system, the researchers measured something once thought to be impossible—paired electrons sharing an orbital seemed to have a combined spin under dark conditions, suggesting a forbidden 'triplet' state when instead their spins should cancel one another out.